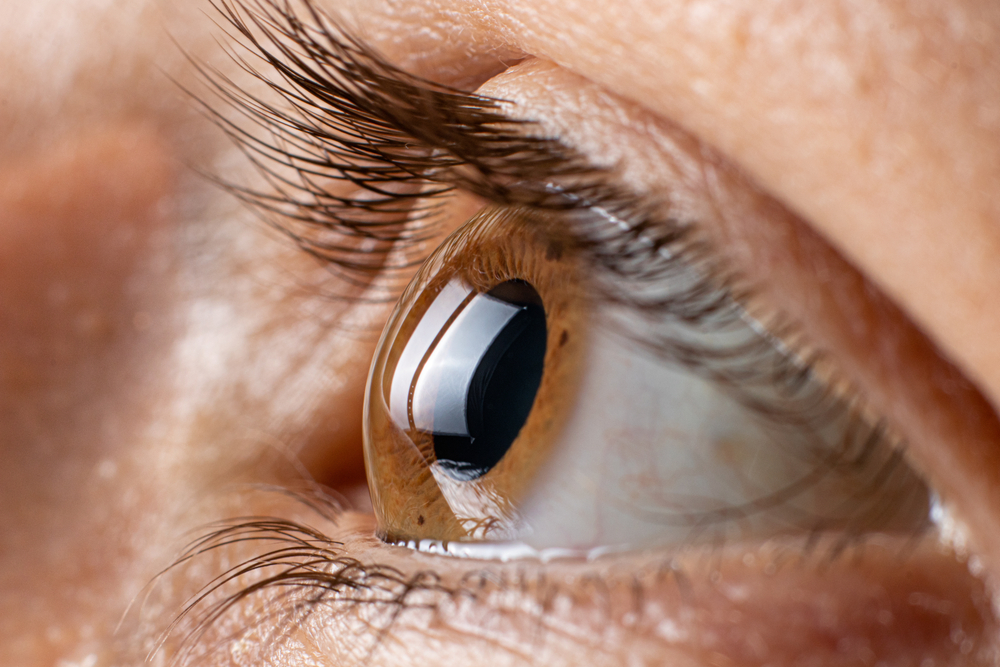
Recent studies have intensified concerns regarding the potential side effects of Ozempic and Wegovy, two widely-used medications prescribed for diabetes and weight management. Specifically, new research published this month highlights a troubling association between these drugs and a rare but serious eye condition known as non-arteritic anterior ischemic optic neuropathy (NAION). This condition can lead to sudden and often irreversible vision loss, raising alarm among healthcare professionals and patients alike.
Ozempic and Wegovy Uses
Ozempic and Wegovy are both medications that contain the active ingredient semaglutide, which belongs to a class of drugs known as glucagon-like peptide-1 receptor agonists (GLP-1 RAs). Initially approved for the management of type 2 diabetes, Ozempic has gained popularity due to its significant weight loss effects, prompting the development of Wegovy, specifically marketed for obesity management.
Semaglutide works by mimicking a hormone that regulates appetite and insulin levels, thereby helping to control blood sugar levels and promote weight loss. While these benefits have made Ozempic and Wegovy popular choices among patients, the recent findings regarding potential side effects have raised important questions about their safety.
Since their introduction, Ozempic and Wegovy have been prescribed to millions of individuals. However, the increasing awareness of their side effects, particularly concerning vision health, has led to a growing scrutiny regarding their long-term use.
Alleged Ozempic Vision Loss Risks
A recent study published on December 11 in medRxiv has established a strong correlation between the use of semaglutide and the risk of developing NAION or non-arteritic anterior ischemic optic neuropathy. This eye condition occurs when there is insufficient blood flow to the optic nerve, leading to sudden vision loss.
Researchers from Denmark and Norway analyzed data from over 60,000 users of Ozempic and Wegovy, comparing them to patients taking other diabetes medications. The results indicated that those using semaglutide faced more than double the risk of developing NAION compared to those on alternative treatment regimens.
The implications of these findings are significant, particularly for patients with type 2 diabetes who are already at a higher risk for vision-related complications. The study estimated an increase of 1.41 NAION events per 10,000 person-years among users of Ozempic and Wegovy.
Previous Ozempic Research and Side Effect Concerns
Concerns about the potential vision-related side effects of Ozempic and Wegovy are not new. Earlier studies, including one conducted by Harvard researchers, indicated that patients using these medications had a substantially higher risk of experiencing vision changes compared to those on different diabetes treatments.
The Harvard study found that patients taking semaglutide had more than seven times the risk of experiencing significant vision changes. This alarming statistic has prompted further investigation into the safety profile of these medications.
In response to these findings, Danish health authorities have requested a review from the European Union’s drug regulator. They aim to assess the implications of the studies linking Ozempic to NAION, further emphasizing the need for a thorough evaluation of the safety of these drugs.
Gastrointestinal Issues Allegedly Linked to Semaglutide
In addition to vision concerns, patients taking Ozempic and Wegovy have reported various gastrointestinal problems. These side effects include conditions such as gastroparesis (commonly known as stomach paralysis), ileus, and intestinal blockages, which can significantly impact a patient’s quality of life.
Gastroparesis is a condition characterized by delayed gastric emptying, leading to symptoms such as nausea, vomiting, and abdominal pain. Patients who experience these issues may find it challenging to manage their diabetes or maintain a healthy weight.
The emergence of these potential gastrointestinal complications has raised questions about the adequacy of the warnings provided by Novo Nordisk. Patients deserve to be fully informed about the potential risks associated with their medications so they can make educated choices about their treatment options.
A Growing Number of Ozempic Injury Lawsuits
As more evidence surfaces regarding the potential risks associated with Ozempic and Wegovy, legal actions against Novo Nordisk are gaining momentum. The consolidation of lawsuits in the U.S. District Court is a significant development, as it allows for coordinated pretrial proceedings. U.S. District Judge Karen S. Marston is overseeing the litigation, focusing on critical issues that may affect numerous claims. This includes examining the general causation evidence regarding the alleged link between semaglutide and various injuries, including NAION and gastrointestinal complications.
Ozempic Lawsuit Information
Ozempic Gastroparesis Lawsuits, Leading Justice
Risk of Nonarteritic Anterior Ischemic Optic Neuropathy in Patients Prescribed Semaglutide, JAMA Ophthalmology
Ozempic Lawsuits Soar as New Vision Loss Risks Emerge, Leading Justice