A new Suboxone lawsuit filed last week in the U.S. District Court of Western Pennsylvania highlights a concerning issue regarding the widely used anti-opioid drug – the lack of warnings about tooth decay and other dental issues. In recent years, the use of Suboxone, a medication containing buprenorphine and naloxone, has become increasingly prevalent in the treatment of opioid addiction. However, in this latest product liability lawsuit, brought against Indivior, Reckitt Benckiser, and Aquestive Therapeutics, plaintiff Lindsay Haddad alleges that this omission placed users at unnecessary risk of severe and potentially permanent dental injuries.
What is Suboxone Used to Treat?
Suboxone, which contains the medications buprenorphine and naloxone, is a medication commonly prescribed to individuals undergoing treatment for opioid addiction. It works by reducing withdrawal symptoms and cravings, providing relief and support during the recovery process. Suboxone is available in various forms, including tablets and sublingual films. The sublingual films, dissolved under the tongue or against the cheek, offer a convenient method of administration.
The Risk of Suboxone Film Tooth Decay
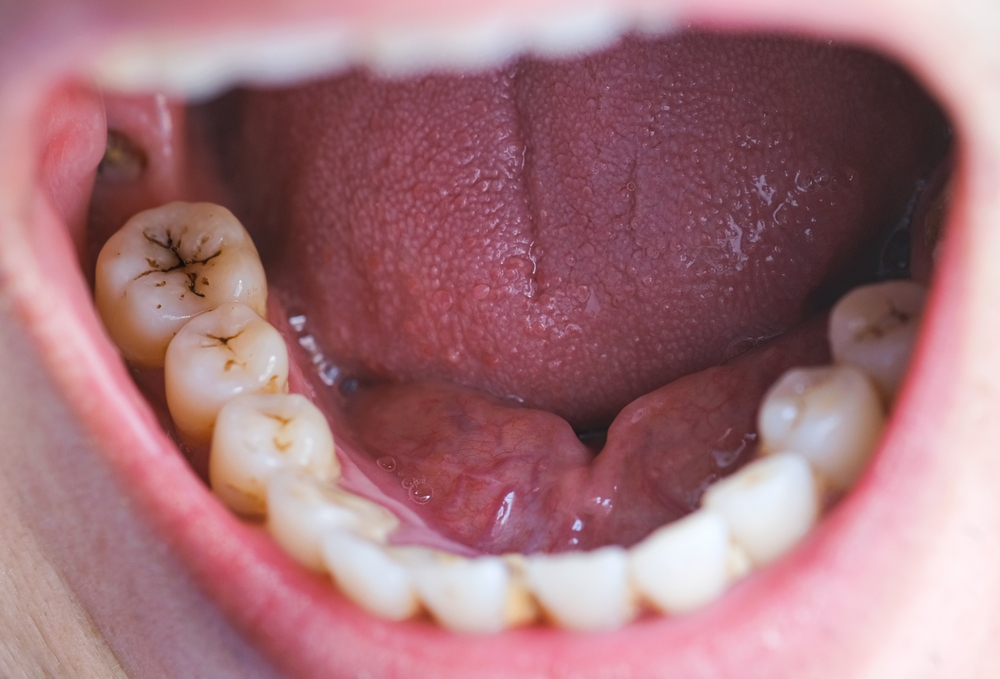
Despite the benefits of Suboxone in treating opioid use disorder, concerns have emerged regarding its potential for causing dental problems. In January 2022, the U.S. Food and Drug Administration (FDA) issued a warning highlighting reports of dental issues associated with buprenorphine-containing medicines dissolved in the mouth, including Suboxone. These issues include tooth decay, cavities, oral infections, and even tooth loss. It is important to note that these problems have been reported in individuals with no prior history of dental issues, amplifying the significance of the link between Suboxone film and tooth decay.
The Suboxone Lawsuit by Lindsay Haddad
Lindsay Haddad’s Suboxone lawsuit alleges that the lack of warnings and instructions regarding Suboxone film tooth decay placed users at unnecessary risk. Haddad claims that had she been adequately informed about the potential dental risks associated with Suboxone film, she could have taken preventive measures to avoid tooth damage. Haddad’s lawsuit seeks both compensatory and punitive damages, highlighting the severity of the dental injuries she suffered and the potential impact on her life.
Research Studies on Suboxone and Tooth Damage
Several research studies have explored the link between Suboxone, specifically the sublingual film form of the drug, and tooth damage. One study published in the journal Primary Care Companion for CNS Disorders found that the acidity of Suboxone films can contribute to the erosion of tooth enamel, leading to tooth decay and cavities. The prolonged exposure of teeth to Suboxone films, which can take up to 10 minutes to dissolve, further increases the risk of dental issues. “The prolonged contact between tooth surfaces with buprenorphine/naloxone […] may be a contributing factor in the alteration of tooth surface microbial profile and/or the pH to promote dental caries,” the researchers reported, “similar to what has been previously reported in patients who use methamphetamine.”
In its January 2022 warning, the FDA reported that a search of the agency’s Adverse Event Reporting System database and the medical literature through December 2018 revealed “305 cases of dental adverse events reported with transmucosal buprenorphine use.” According to the FDA, “Patients with opioid use disorder (OUD) may have a higher incidence of poor dental health; however, many cases described severe dental issues in patients with no reported prior history of dental problems.” These findings emphasize the need for comprehensive warnings and dental care recommendations for individuals using Suboxone film.
The Importance of Dental Health in Suboxone Treatment
Dental care plays a crucial role in a patient’s overall health during Suboxone treatment. It is essential for individuals using Suboxone films to take proactive steps to reduce the risk of tooth decay and other dental problems. Proper administration techniques, such as following the recommended dissolving instructions and rinsing the mouth after dissolution, can help minimize the contact between Suboxone and teeth. Additionally, individuals should wait at least an hour before brushing their teeth to avoid causing further damage to the softened enamel.
Regular dental check-ups and communication with healthcare professionals are also vital for individuals using Suboxone films. Dental professionals can assess the oral health of patients and detect any early signs of tooth decay or other dental issues. It is important to inform dentists about the use of Suboxone films and any history of dental problems. By working closely with both doctors and dentists, individuals can ensure that their dental health is monitored and any potential problems are addressed promptly.
The Future of Suboxone Film Tooth Decay Lawsuits
Lindsay Haddad’s Suboxone lawsuit joins a growing number of complaints against the manufacturers of Suboxone. These lawsuits aim to hold the drug manufacturers accountable for their alleged failure to provide adequate warnings and instructions regarding the dental risks associated with Suboxone film use. The U.S. Judicial Panel on Multidistrict Litigation is currently reviewing the cases to determine whether they should be consolidated and heard before one judge for coordinated pretrial proceedings. This consolidation would streamline the legal process and facilitate consistent rulings on common issues.
Suboxone Lawsuit Information
FDA warns about dental problems with buprenorphine medicines dissolved in the mouth to treat opioid use disorder and pain, FDA