The opioid crisis has devastated communities across America, leaving countless individuals struggling with addiction and its debilitating consequences. In the midst of this public health emergency, a medication called Suboxone emerged as a treatment option to help those battling opioid dependence. However, a growing number of Suboxone users are now seeking legal recourse, alleging that the manufacturers of this drug failed to adequately warn them about a potentially severe side effect – catastrophic tooth decay.
Over 9,600 plaintiffs have now joined a consolidated Suboxone lawsuit, filing their claims just ahead of a critical deadline that could have barred many from seeking justice. This mass filing represents a significant escalation in the legal battle against the makers of the opioid addiction drug, underscoring the widespread and devastating impact of Suboxone’s dental side effects.
The Opioid Epidemic and the Rise of Suboxone
The opioid crisis in the United States has been a public health emergency of staggering proportions. Fueled by the aggressive marketing and distribution of highly addictive prescription painkillers by pharmaceutical companies, opioid abuse has claimed the lives of nearly 800,000 people since 1999. In response to this crisis, the FDA approved Suboxone in 2002 as a medication-assisted treatment (MAT) option to help individuals overcome opioid addiction.
Suboxone, a combination of the synthetic opioid buprenorphine and the opioid antagonist naloxone, was intended to reduce cravings and manage withdrawal symptoms for those struggling with opioid dependence. The drug was initially available in a dissolvable tablet form, but about a decade later, the manufacturers introduced a sublingual film version, which they claimed was a safer and more convenient alternative.
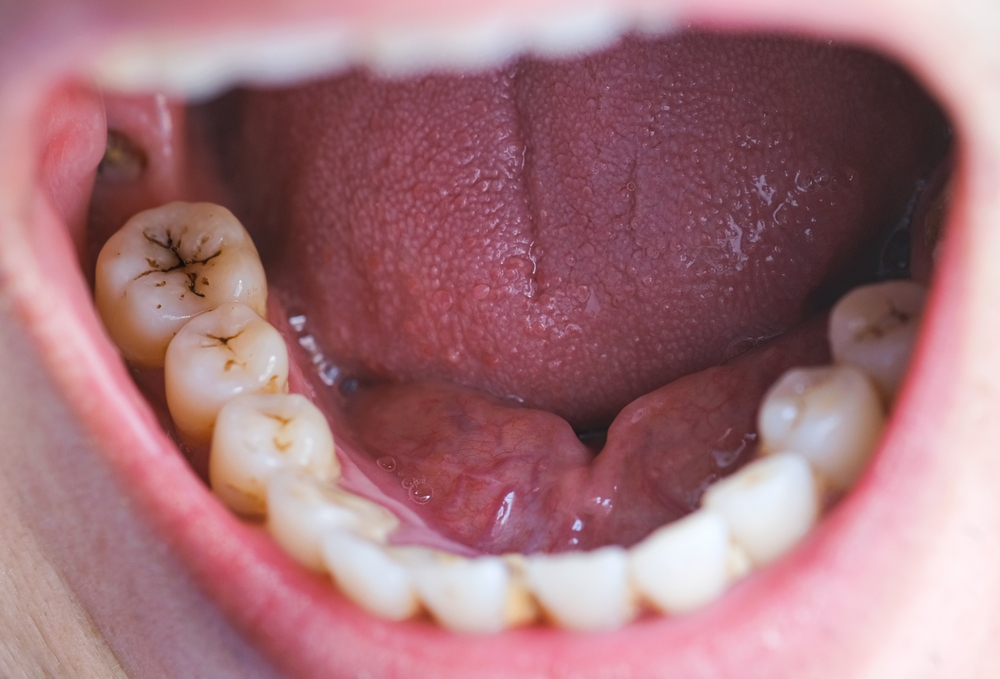
The Dangers of Suboxone Film: Tooth Decay and Dental Damage
However, the introduction of the Suboxone film has been plagued with controversy. Lawsuits allege that the film version was developed not to improve patient outcomes, but rather to delay competition from generic versions of the original tablet and maintain the drug’s profitability. More importantly, these lawsuits assert that the manufacturers failed to adequately warn consumers and healthcare providers about the potential for Suboxone film to cause severe tooth decay and other devastating dental problems.
Numerous studies and case reports have documented the link between Suboxone use, particularly the film formulation, and a range of oral health issues. Patients have reported experiencing cavities, tooth loss, tooth fractures, gum infections, and other dental complications while using the medication. In many cases, these individuals have required extensive and costly dental treatments, such as extractions, root canals, and restorative procedures.
The FDA’s Belated Warning and the Surge of Suboxone Lawsuits
It was not until June 2022, over a decade after the Suboxone film was introduced, that the FDA finally required the manufacturers to add a warning about the potential for dental problems to the medication’s label. This belated action came after the agency had received more than 300 reports of adverse events related to tooth decay and other oral health issues associated with Suboxone use.
Plaintiffs in the Suboxone lawsuits argue that if earlier warnings had been provided, they may have been able to take preventive measures to protect their teeth, or even avoided the use of the medication altogether, thereby preventing the permanent damage they have suffered. The failure of the manufacturers to proactively address this issue and inform consumers has now led to a flood of legal actions, with the number of plaintiffs growing exponentially in a short period of time.
The Suboxone Product Liability Multidistrict Litigation
In response to the growing number of Suboxone-related tooth decay lawsuits filed across the country, the U.S. Judicial Panel on Multidistrict Litigation (JPML) decided to centralize all claims in a single federal court. In February 2024, the JPML consolidated the existing Suboxone cases in the Northern District of Ohio, under the oversight of U.S. District Judge Philip Calabrese.
The Suboxone MDL has seen a remarkable surge in the number of plaintiffs joining the legal battle. While the initial filing in late 2023 involved around 20 cases, the latest mass filing has added more than 9,600 individuals to the litigation, dramatically expanding the scope and potential liability faced by the defendants.
Battling the Suboxone Injury Statute of Limitations
The rapid influx of new Suboxone lawsuits is largely driven by a looming deadline that could have barred many potential plaintiffs from seeking compensation. The manufacturers have argued that the two-year anniversary of the FDA’s label update, which occurred in June 2022, may trigger the statute of limitations for some claims in certain states.
Recognizing the potential for thousands of individual complaints to be filed in a short timeframe, the presiding judge, Judge Calabrese, issued a case management order allowing plaintiffs to submit a bundled Suboxone lawsuit by June 14, 2024. This decision has enabled an unlimited number of individuals to join the litigation, ensuring that those who may have been impacted by the dental side effects of Suboxone can have their voices heard and their claims addressed.