The opioid epidemic has emerged as a significant public health crisis, affecting millions globally. This widespread issue is characterized by the overprescription of opioid medications, leading to addiction and tragic overdoses. In response to this crisis, various medications have been developed to assist individuals in overcoming opioid dependence. One such medication is Suboxone, a combination of buprenorphine and naloxone, which has been both praised for its effectiveness and criticized for its potential risks.
What is Suboxone?
Suboxone is a drug primarily utilized in addiction treatment programs to help individuals manage withdrawal symptoms and cravings associated with opioid use disorder. Its dual-action mechanism allows it to alleviate pain while also preventing the euphoric effects that can lead to misuse. Despite its intended benefits, Suboxone has recently become the center of numerous lawsuits, raising questions about its safety, marketing practices, and the responsibilities of pharmaceutical companies.
How Suboxone Works in Addiction Treatment
Suboxone consists of two active ingredients: buprenorphine and naloxone. Buprenorphine is a partial agonist that activates opioid receptors in the brain, providing relief from withdrawal symptoms without producing a full opioid effect. This feature is crucial in minimizing the risk of addiction. Naloxone, on the other hand, acts as an opioid antagonist, blocking the effects of opioids if the drug is misused by injection, thereby deterring potential abuse.
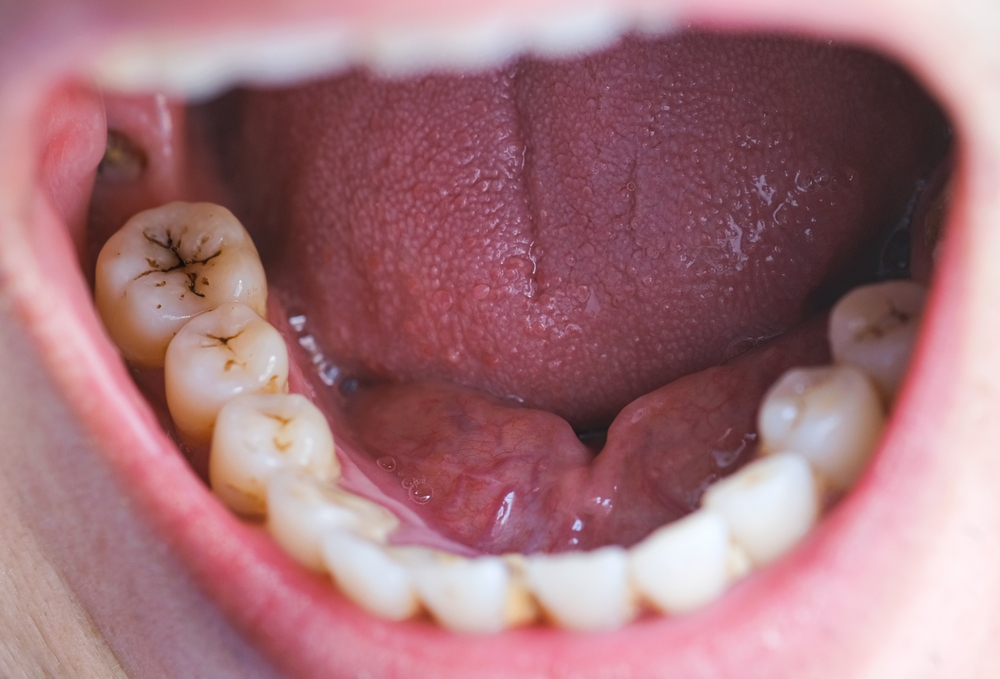
The combination of these two components allows Suboxone to play a vital role in comprehensive addiction treatment plans. By reducing cravings and withdrawal symptoms, Suboxone has enabled many individuals to achieve stable recovery. However, the complexities surrounding its use and the potential for Suboxone tooth decay side effects have led to increasing scrutiny from healthcare professionals, patients, and legal entities alike.
The Rise of Legal Claims Against Suboxone Manufacturers
In recent years, the legal landscape surrounding Suboxone has become increasingly complex. Numerous lawsuits have been filed against pharmaceutical companies responsible for its production, alleging various forms of misconduct. These claims often center on issues such as deceptive marketing practices, inadequate warnings about potential risks, and the overall safety of the drug.
A significant aspect of these lawsuits is the allegation that manufacturers exaggerated the benefits of Suboxone while downplaying its risks. Critics argue that this has contributed to a culture of overprescription, ultimately leading to increased addiction rates among patients who may have been inadequately informed about the drug’s potential dangers.
Key Suboxone Treatment Concerns: Misuse and Alleged Side Effects
Despite the intended benefits of Suboxone, concerns have been raised regarding its potential for misuse. Although it is designed to minimize euphoric effects, some individuals have reportedly abused the drug for its sedative properties. This has prompted discussions about the adequacy of prescribing practices and the responsibilities of healthcare providers in monitoring patient use. Additionally, patients have reported various side effects associated with Suboxone, including but not limited to:
- Tooth decay
- Cavities
- Oral infections
- Tooth loss
- Tooth erosion
- Dental damage
These alleged side effects have raised questions about the overall safety of Suboxone and whether patients are being adequately informed of the potential risks.
The Impact of Aggressive Marketing Strategies
Another critical factor in the ongoing Suboxone lawsuits is the alleged aggressive marketing tactics employed by pharmaceutical companies. Critics assert that these companies have engaged in misleading advertising, which has contributed to the widespread use of Suboxone without sufficient regard for its risks.
These marketing strategies often emphasize the drug’s effectiveness in treating opioid addiction while downplaying the potential for misuse and side effects. Such practices have led to increased scrutiny from regulatory agencies and have fueled public concern regarding the accountability of pharmaceutical manufacturers.
Possible Grounds for Suboxone Lawsuits
The legal claims surrounding Suboxone primarily stem from allegations of improper marketing, product liability, and insufficient risk warnings. Many Suboxone lawsuits assert that pharmaceutical companies failed to adequately disclose the addictive properties of Suboxone, leading to dependency among patients.
Product liability claims often focus on the drug’s design and formulation, with plaintiffs arguing that the manufacturers did not take appropriate steps to ensure the drug’s safety. Furthermore, some lawsuits question the ethical responsibilities of healthcare providers in prescribing Suboxone without proper patient monitoring.
Current Status of Suboxone Lawsuits
As of late 2024, there are numerous ongoing lawsuits related to Suboxone, with many claims focusing on insufficient warnings about the drug’s alleged risks. Reports indicate that there are hundreds of pending lawsuits in various jurisdictions, highlighting the widespread concern over Suboxone’s safety and the practices of its manufacturers.
These legal proceedings have not only raised awareness about the potential dangers associated with Suboxone but have also prompted discussions about the need for stricter regulations governing prescription medications. The outcomes of these lawsuits could significantly impact the future of Suboxone and similar medications used in addiction treatment.
Holding Allegedly Negligent Pharmaceutical Companies Accountable
Pharmaceutical companies are under increasing pressure to be transparent about the risks associated with their drug products. Allegations of misleading marketing and inadequate warnings have raised serious questions about the accountability of these companies in ensuring patient safety.
As Suboxone lawsuits continue to unfold, there is a growing demand for stricter regulations governing pharmaceutical marketing practices. Advocates argue that greater transparency is necessary to protect patients from potential harm and to ensure that healthcare providers are well-informed about the medications they prescribe.
Suboxone Lawsuit Information
Suboxone Tooth Decay Lawsuit, Leading Justice